We empower access to medicines on a global level.
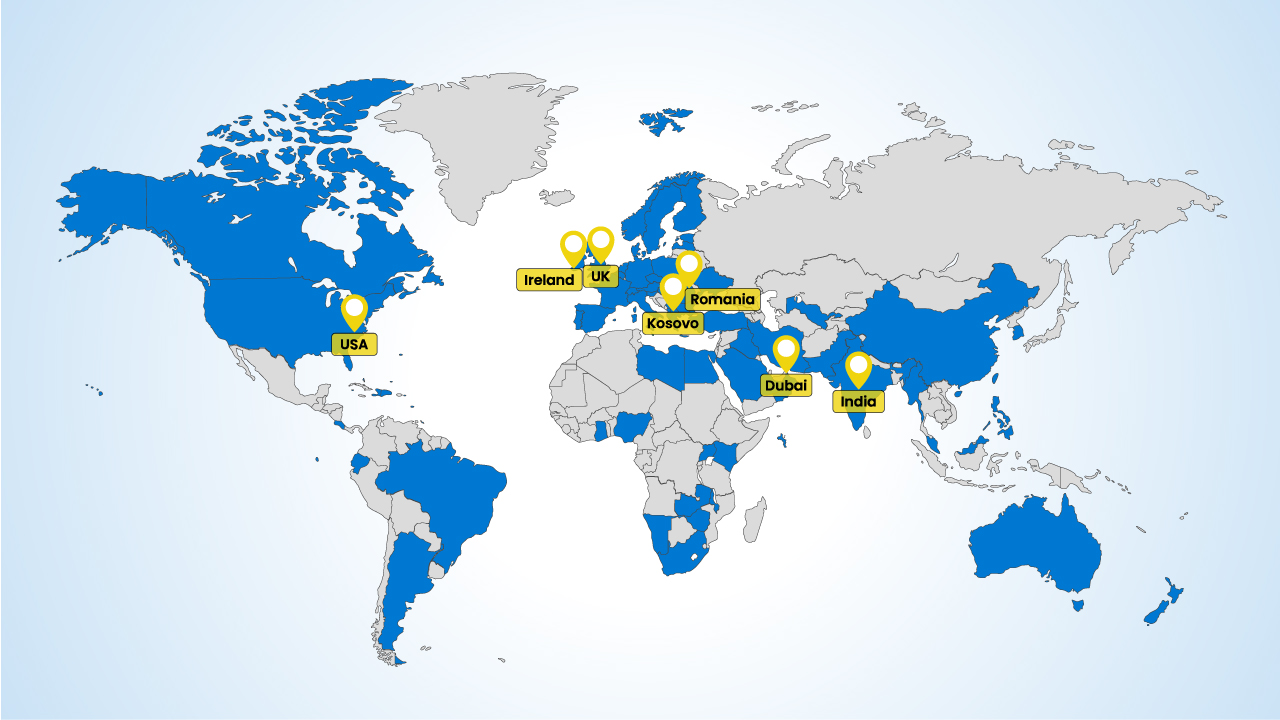
Using our experience, regulatory expertise and network of suppliers from over 25 countries, we provide access to over 87% of the world health system from our distribution hubs in the EU, UK, Dubai, India and more.
We partner with healthcare professionals, pharmaceutical & biotech companies, wholesalers & distributors, aid organizations and patients to ensure access to medicines.
From regulatory analysis to strategic plans, procurement and delivery, we provide best-in-class programs and services.
Over two decades of focused expertise.
Aid & Development
We partner with NGOs, charities and multi-lateral organizations to provide pharmaceuticals, medical disposables, medical equipment and hospital and doctors’ furniture.

Comparator/RLD Supply
We support manufacturers, CROs and CDMOs with supply of comparator, RLDs, samples and biosimilar samples.

Early Access Programs (EAP)
We’ll partner with innovative Pharma and Biotech companies to design and manage global Early Access, Expanded Access, Named Patient and Compassionate Use Programs.

Hospital & Pharmacy Supply
Thanks to our global sourcing network, we supply products that are in shortage, unlicensed medicines, imported medicines, specials, and not yet launched medicines to hospitals, pharmacies and globally.

International Supply & Unlicensed Imports
We source and distribute on a global basis, supplying branded and generic pharmaceuticals not otherwise available in your country. We also supply a range of unlicensed medicines to hospitals and healthcare professionals in the UK, UAE and globally.

Sexual Health Supply
We support UK hospitals and sexual health clinics with pharmaceuticals and medical disposables.

Specialist Distribution
We work with Pharma and Biotech companies to deliver specialist distribution services. We operate in therapeutic and geographic areas that need specialist expertise.
What’s new at Smartway

07 March 2024
News
Supporting Rare Disease Day, 2024Celebrated on 29 February 2024, the rarest day of the year, Rare Disease Day unites the global rare disease community to address challenges and promote equity in healthcare, diagnosis, and treatment accessibility. It's a day to come together to share knowledge and support, aiming t...
10 November 2023
News
Medicine Safety Week 2023: Our Commitment to Patient SafetyMedicines regulators depend on the reporting of adverse drug reactions (ADRs) to ensure the safety of medicines. Unfortunately, however, all reporting systems suffer from underreporting, which may limit regulators’ ability to comprehensively assess medication safety on an ongoing basis. During Medicines Safety Week, medicine regulators and healthcare authorities from across the globe join ...
17 September 2023
News
World Patient Safety Day 2023: Our Pledge to Amplifying Patients' VoicesToday is World Patient Safety Day (WPSD). WPSD was established in 2019 by the 72nd World Health Assembly through the adoption of resolution WHA72.6, entitled "Global Action on Patient Safety". WPSD is about raising awareness of the importance of patient engagement. It also aims to educate, increase awareness and ultimately, help reduce pa...
